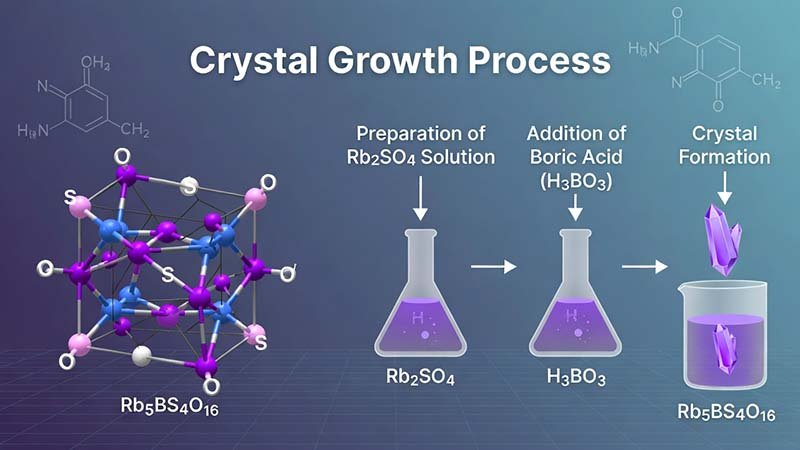
Rubidium Borosulfate (Rb5BS4O16) & Crystal Growth Process — Built Around Rubidium Sulfate (Rb2SO4)
Chemical formula: Rb5BS4O16 · Molecular weight: 822.40 · Crystal system: Tetragonal · Space group: P43212
1) Overview
Rubidium borosulfate Rb5BS4O16 is a boron–sulfate functional material that combines borate and sulfate groups in one lattice. The crystal is tetragonal (space group P43212) and can be grown to large sizes (up to centimeter-class in scale) using a practical two-stage route: (i) solid-state synthesis to obtain phase-pure polycrystalline Rb5BS4O16 powder, followed by (ii) flux-assisted high-temperature melt growth to produce single crystals.
For manufacturers targeting stable quality and repeatable crystal growth, rubidium sulfate (Rb2SO4) is the anchor feedstock: it supplies Rb and sulfate in one material, keeps sulfur stoichiometry on target, and supports cleaner conversion during the solid-state stage.
2) Detailed Production Process & Practical Summary
A. Solid-state reaction → phase-pure Rb5BS4O16 polycrystalline powder
B. Flux melt preparation (PbO / H3BO3 / PbO–H3BO3) → mixed melt
C. Seed formation → seed crystal acquisition
D. Controlled growth → large single crystal
Solid-state: 400 °C (12 h) → regrind → 500 °C (48 h)
Melt: 480–600 °C (12–100 h) depending on recipe
Seed: cool to 440–550 °C, dip/withdraw, then cool to RT
Growth: 0.1–3 °C/day slow cooling, rotation 0–100 rpm
A) Solid-State Synthesis of Rb5BS4O16 (Phase-Pure Powder)
-
Raw material selection (molar basis Rb : B : S = 5 : 1 : 4).
Recommended to center the recipe around Rb2SO4 as the primary rubidium + sulfate provider.
- Rubidium sources (choose one or combine): Rb2SO4, Rb2O, RbNO3, Rb2CO3, or RbOH
- Sulfur sources: Rb2SO4 or (NH4)2SO4
- Boron sources: H3BO3 or B2O3
- Weighing & mixing: weigh reactants to the required molar ratio; grind thoroughly in a mortar to ensure homogeneity.
- First calcination: transfer the homogenized mixture into a corundum crucible; heat slowly to 400 °C, hold for 12 hours, then cool to room temperature.
- Intermediate regrind: remove the product, grind again to break aggregates and improve diffusion contact.
- Second calcination: return to the crucible; heat to 500 °C, hold for 48 hours, then cool.
- Final milling: grind carefully to obtain single-phase polycrystalline Rb5BS4O16 powder.
- Phase confirmation (recommended QC): verify phase purity by XRD; the pattern should match the Rb5BS4O16 reference.
Typical Solid-State Reaction Sets (Examples)
| Example reactant set | What Rb2SO4 contributes | Gas evolution note |
|---|---|---|
| 5Rb2SO4 + 3(NH4)2SO4 + 2H3BO3 → 2Rb5BS4O16 + 6NH3↑ + 6H2O↑ | Primary rubidium + sulfate backbone; stabilizes sulfur stoichiometry. | Mainly NH3/H2O from ammonium salt & boric acid dehydration. |
| 5Rb2CO3 + 8(NH4)2SO4 + 2H3BO3 → 2Rb5BS4O16 + 16NH3↑ + 11H2O↑ + 5CO2↑ | Rb2SO4 can replace carbonate in equivalent Rb supply while reducing CO2 evolution complexity. | CO2 present if using carbonate; more off-gas handling needed. |
| 20RbNO3 + 16(NH4)2SO4 + 4H3BO3 → 4Rb5BS4O16 + 20NO2↑ + 22H2O↑ + 32NH3↑ + 5O2↑ | Switching to Rb2SO4 avoids nitrate-derived NOx streams and simplifies EHS. | NOx and O2 can appear with nitrate routes; stricter abatement required. |
B) Flux Melt Preparation (High-Temperature Melt Route)
After obtaining phase-pure Rb5BS4O16 powder, prepare a flux melt that supports stable crystal growth.
-
Blend compound + flux: mix Rb5BS4O16 powder with a flux system such as
PbO, H3BO3, or PbO–H3BO3.
- Molar ratio guideline: Rb5BS4O16 : flux = 1 : 1 to 6
- For PbO–H3BO3 system: PbO : H3BO3 = 1–2 : 1–5
- Heat to form a mixed melt: using a ramp rate of 1–80 °C/h, heat to 480–600 °C, then hold for 12–100 hours to obtain a homogeneous mixed melt.
- One-pot option: alternatively, charge rubidium source + sulfur source + boron source together with flux in one crucible, then heat at 1–80 °C/h to 480–500 °C, hold 12–24 hours to form the mixed melt directly.
C) Seed Crystal Acquisition
- Cool the melt: reduce melt temperature to 440–550 °C.
- Dip the seed rod: insert the seed rod below the melt surface.
- Controlled drop for nucleation: lower temperature at 0.5–5 °C/h for a total of 10–30 °C.
- Withdraw & consolidate: pull the rod above the surface; deposits/aggregates appear on the rod.
- Cool to room temperature: cool down at 1–80 °C/h to obtain Rb5BS4O16 seed crystals.
D) Bulk Crystal Growth (Flux Growth)
- Set growth window: cool the mixed melt to 430–540 °C.
- Mount seed: fix the seed from step C onto the seed rod; lower from the top of the growth furnace.
- Preheat seed: preheat for 5–60 minutes to reduce thermal shock.
- Touch & re-melt: bring the seed to contact the melt surface (or immerse) for re-melting; hold 5–60 minutes.
- Fast settle into growth temperature: quickly cool to 420–530 °C.
- Slow cooling growth: cool at 0.1–3 °C/day while rotating the seed rod at 0–100 rpm to control mass transport and facet development.
- End of growth: once the target size is reached, lift the crystal out of the melt.
- Controlled cool-down: cool to room temperature at 1–80 °C/h, then remove the crystal.
Process Summary (What This Route Delivers)
- Fast and practical: solid-state stage is straightforward; flux growth shortens overall growth cycle.
- Scalable crystal size: supports large single crystals (commonly mm-scale; can be extended to cm-class with tuned thermal fields and flux ratios).
- Machinability: crystals show good mechanical performance and are easier to process compared with many brittle optical salts.
- Wide transmission potential: material architecture (borate + sulfate groups) supports broad optical transparency design space.
3) Why Rubidium Sulfate (Rb2SO4) Matters — Advantages of This Feedstock + Process
A) Rb2SO4 is the Most “Direct” Rb + Sulfate Source
- Two roles in one: Rb2SO4 supplies both Rb and the sulfate framework, which helps lock in the target Rb:B:S stoichiometry (5:1:4) early in the solid-state stage.
- Cleaner conversion profile: compared with nitrate or carbonate rubidium salts, Rb2SO4-based routes reduce unwanted side streams (e.g., NOx from nitrates, extra CO2 from carbonates), simplifying gas management and improving batch reproducibility.
- Stable, non-volatile sulfate feed: sulfate chemistry supports consistent sulfur delivery during heating and regrinding cycles, which is critical for obtaining phase-pure Rb5BS4O16 powder before crystal growth.
B) Better Reproducibility = Better Crystals
- Phase purity is everything: flux growth performance depends strongly on a stable, phase-pure starting powder. Rb2SO4 helps stabilize the “sulfate budget,” improving the chance of single-phase Rb5BS4O16.
- More controllable melt chemistry: consistent sulfate input reduces melt composition drift, making it easier to hit the right nucleation and growth window (440–550 °C for seed handling; 0.1–3 °C/day for controlled growth).
- Optical-grade impurity control: for functional crystals, trace alkali impurities (Na/K), insolubles, and moisture can matter. A high-purity Rb2SO4 feedstock improves downstream optical uniformity and processing yield.
C) Manufacturer’s Perspective: What to Specify for Rb2SO4 When Targeting Crystal Growth
- Purity level: select an assay aligned with optical-grade synthesis goals; control trace Na/K and insoluble residues.
- Moisture management: request controlled moisture / loss-on-drying to stabilize weighing accuracy and batch-to-batch stoichiometry.
- Particle size distribution (PSD): consistent PSD improves mixing homogeneity and solid-state reaction kinetics during the 400 °C → 500 °C two-step firing.
- Packaging: moisture-barrier packaging (sealed inner bag + protective outer) supports stable storage and repeatable production.