Rubidium Carbonate (Rb₂CO₃)
High Purity Rubidium Carbonate Manufacturer & Supplier
Synonyms: Carbonic acid dirubidium, Dirubidium carboxide, Rubidium Salt
CAS Number: 584-09-8 , EC Number:209-530-9
Brand Name : DQ
Percentage assay:99.5%
Package Information: Bottle or 25kg/drum or customized packing
Table of Contents
Product Overview
Rubidium Carbonate (Rb₂CO₃) is a high-purity inorganic compound widely used in optical crystals, electro-optic materials, specialty glass, and advanced research applications.
As a professional manufacturer, we supply Industrial-grade and Electronic-grade rubidium carbonate with strict impurity control, suitable for demanding industrial and R&D environments.
Our Rubidium Carbonate is produced under controlled synthesis and purification processes to ensure consistent quality, high chemical stability, and excellent batch-to-batch reproducibility.
Packaging & Logistics
- Packaging options: 1 kg / 5 kg / 10 kg / customized
- Inner packaging: moisture-proof sealed bags
- Outer packaging: fiber drum or carton
- Delivery: air / sea shipment available
- Documentation: COA, MSDS, TDS provided
Rubidium carbonate Price
We always offer our high-quality products at very competitive prices! The exact pricing depends on factors such as purity grade, trace metal limits, packaging specifications, and shipping destination.
Product Information
Impurity Content (Mass Fraction)/%, Not Exceeding
| Product Grade | Li | Na | K | Cs | Ca | Mg | Fe | Al | SiO2 | Pb |
|---|---|---|---|---|---|---|---|---|---|---|
| 99.0% | 0.0010 | 0.020 | 0.050 | 0.50 | 0.0050 | 0.0010 | 0.0010 | 0.0050 | 0.0050 | 0.0010 |
| 99.5% | 0.0010 | 0.010 | 0.020 | 0.20 | 0.0050 | 0.0010 | 0.0005 | 0.0050 | 0.0050 | 0.0005 |
| 99.9% | 0.0005 | 0.0050 | 0.0080 | 0.50 | 0.0010 | 0.0005 | 0.0005 | 0.0010 | 0.0050 | 0.0005 |
| Parameter | Details | Units | Applications |
|---|---|---|---|
| Chemical Formula | Rb₂CO₃ | N/A | Chemistry, Research |
| Molecular Weight | 230.94 | g/mol | Laboratory |
| Appearance | White, hygroscopic powder | N/A | Glass Manufacturing |
| Melting Point | Decomposes at 837 | °C (1,539°F) | Industrial Processes |
| Boiling Point | Decomposes | N/A | N/A |
| Density | 3.2 | g/cm³ | Material Science |
| Solubility in Water | 186 | g/100 mL at 20°C | Chemical Solutions |
| Solubility in Other Solvents | Insoluble | N/A | N/A |
| pH | Alkaline | N/A | Water Treatment |
| Crystal Structure | Orthorhombic | N/A | Crystallography |
| Thermal Conductivity | Low | N/A | Thermal Management |
| Electrical Conductivity | Low | N/A | Electronics |
| Common Uses | Glass, catalyst | N/A | Various Industries |
| Hazards | Irritant | N/A | Safety Precautions |
Rubidium Carbonate Formul
| Reaction Type | Reactants | Products | Balanced Equation |
|---|---|---|---|
| Formation | 2 RbOH, CO₂ | Rb₂CO₃, H₂O | 2RbOH(aq)+CO2(g)→Rb2CO3(s)+H2O(l) |
| Decomposition | Rb₂CO₃ | 2 RbOH, CO₂ | Rb2CO3(s)→2RbOH(aq)+CO2(g) |
| Acid Reaction | Rb₂CO₃, 2 HCl | 2 RbCl, CO₂, H₂O | Rb2CO3(s)+2HCl(aq)→2RbCl(aq)+CO2(g)+H2O(l) |
| Solubility | Rb₂CO₃, H₂O | Rb₂CO₃(aq) | Rb2CO3(s)→Rb2CO3(aq) |
Applications of Rubidium Carbonate
| Application Area | Specific Use |
|---|---|
| Electronics | Used in the manufacturing of specialty glass for fiber optic components |
| Chemical Industry | Used as a catalyst in various chemical reactions |
| Biomedicine | Used in pharmaceuticals for certain treatments (though rare) |
| Energy | Used in magnetohydrodynamic (MHD) power generation |
| Research & Development | Used in laboratories for various types of research, including material science |
| Specialty Glass | Used in the production of special types of glass that have unique optical or structural properties |
| Telecommunications | Used in the production of certain types of optical fiber |
| Catalyst | Used to accelerate specific chemical processes |
Rubidium carbonate shines in the production of specialty glass, particularly lithium silicate glass ceramics for dental use. According to patent data (Lithium silicate glass ceramic and glass with rubidium oxide content), Rb₂O content in these glasses ranges from 3.0 to 9.0 wt.%. Here’s a closer look:
- Example 1: At 7.7 wt.% Rb₂O, you’d use about 9.51 grams of Rb₂CO₃ per 100 grams of glass.
- Example 2: For 3.7 to 7.4 wt.% Rb₂O, the range is roughly 4.5 to 9.1 grams per 100 grams of glass.
How do we figure this out? Rb₂O has a molecular weight of about 186.94 g/mol, while Rb₂CO₃ is around 230.94 g/mol. So, 1 gram of Rb₂O needs about 1.235 grams of Rb₂CO₃. For 7.7 grams of Rb₂O, that’s approximately 9.51 grams of rubidium carbonate—pretty straightforward!
Rubidium carbonate is a handy promoter in catalysts, especially for synthesizing higher alcohols. Studies show that in rubidium-promoted iron catalysts, the Rb/Fe atomic ratio ranges from 1.44/100 to 5/100 (Fischer–Tropsch Synthesis: Characterization Rb Promoted Iron Catalyst). Let’s break it down:
- With Rb at 85.47 g/mol and Fe at 55.85 g/mol, an Rb/Fe ratio of 1.44/100 gives about 2.16 wt.% rubidium, or roughly 2.92 grams of Rb₂CO₃ per 100 grams of catalyst (since rubidium is 74% of Rb₂CO₃’s weight).
- At 5/100, it’s about 7.1 wt.% rubidium, or around 9.6 grams of Rb₂CO₃ per 100 grams.
Other research, like “Influence of Cobalt on Rubidium-Promoted Alumina-Supported Molybdenum Carbide Catalysts for Higher Alcohol Synthesis from Syngas” (link), mentions similar uses but doesn’t specify amounts, so we estimate a similar range.
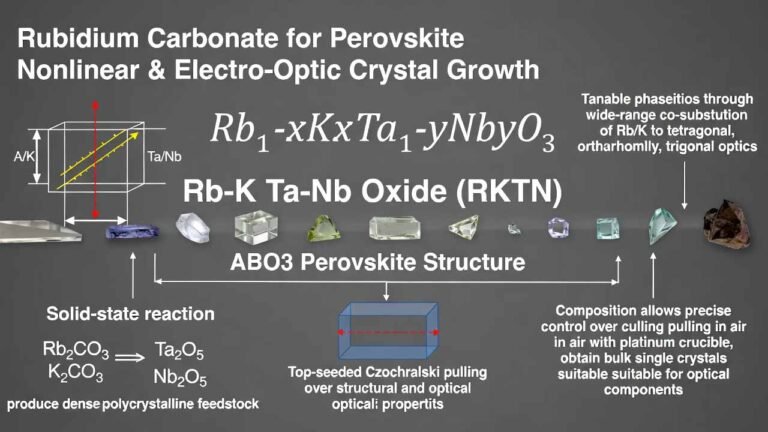
Rubidium titanyl phosphate crystal, with the chemical formula RbTiOPO₄ (abbreviated as RTP), is an isomorph of KTP. RTP crystals have a transmission range of 350–4500 nm, high dielectric constant (ε = 11), high resistivity (approximately 10¹¹–10¹² Ω·cm), high laser damage threshold (1.8 times that of KTP), and extremely low piezoelectric ringing effect. These properties make it highly suitable for electro-optic modulation in lasers, including Q-switches, electro-optic shutters, phase modulators, pulse pickers, and cavity dumpers.
RTP is a biaxial crystal. To eliminate the influence of ambient temperature changes on the refractive index, RTP electro-optic devices typically use two crystals of identical length and performance parameters, with their optical axes oriented perpendicular to each other. This dual-crystal configuration allows stable operation in environments from -50°C to +70°C (provided the temperature field of both crystals remains consistent). Additionally, the series connection of the two crystals halves the modulation voltage, making it particularly suitable for laser rangefinders and medical lasers.
I. Summary of the Fabrication Process
This technology consists of two core parts: (1) preparation of blue-emitting perovskite quantum dot materials and (2) assembly of electroluminescent devices.
1) Preparation of Blue Perovskite Quantum Dots (CsRbₓPbSnᵧ(Br/Cl)₃) (Room-Temperature Synthesis)
The key is to realize co-doping of rubidium (Rb⁺) and tin (Sn²⁺) at room temperature, and then obtain pure blue emission via halide anion exchange. The main procedure is as follows:
Step 1: Prepare the Rb–Cs precursor solution- Dissolve a cesium source (e.g., cesium carbonate) and a rubidium source (rubidium carbonate or rubidium acetate) in oleic acid.
- Heat and stir at 100–150 °C until the solution becomes clear, yielding a mixed precursor containing Rb⁺ and Cs⁺.
- Dissolve lead bromide (PbBr₂), tin(II) bromide (SnBr₂), and a surfactant (tetra-n-octylammonium bromide) together in toluene.
- This step introduces Sn²⁺ as the B-site co-dopant.
- Inject the Rb–Cs precursor solution from Step 1 into the Sn–Pb precursor solution from Step 2 and stir at room temperature.
- Add another surfactant (didodecyldimethylammonium bromide) to trigger nucleation and growth, producing a crude solution of perovskite QDs co-doped with Rb⁺ and Sn²⁺.
- Add an antisolvent (e.g., ethyl acetate) to the crude solution and centrifuge to collect the precipitate.
- Redisperse the precipitate in a non-coordinating solvent (e.g., toluene) to obtain green-emitting QDs.
- Add a toluene solution containing an ammonium chloride salt (e.g., didodecyldimethylammonium chloride) to the green QDs to perform Cl⁻/Br⁻ exchange.
- Precipitate, centrifuge, and purify again, then redisperse in solvent to obtain the target pure-blue perovskite quantum dots.
2) Fabrication Process of the Electroluminescent Device (LED)
The device adopts a multilayer thin-film structure: Anode / Hole injection layer / Hole transport layer / Quantum-dot emissive layer / Electron transport layer / Electron injection layer / Cathode. Key processing steps:- Use spin-coating to form the organic/solution-processed layers (hole injection layer PEDOT:PSS, hole transport layer, and QD emissive layer).
- Use vacuum thermal evaporation to deposit the organic/metal layers (electron transport layer TPBi, electron injection layer LiF, and Al cathode).
- A CuSCN/TFB bilayer hole transport structure is used to optimize hole injection balance.
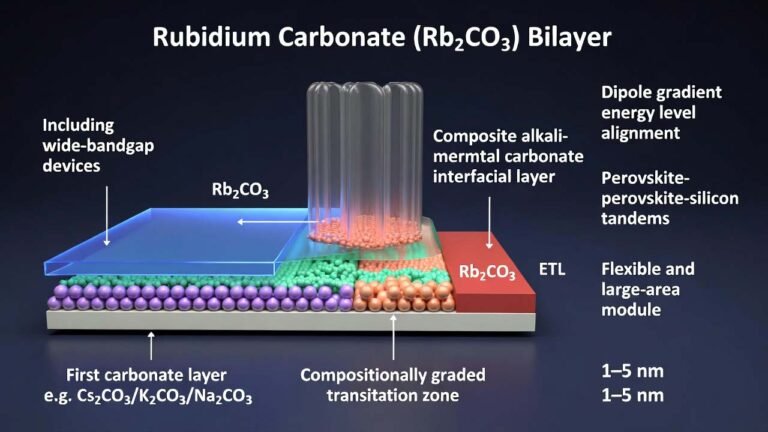
II. Advantages of Adding Rubidium Carbonate / Rubidium Acetate (Enabling Rb Doping)
Using rubidium carbonate or rubidium acetate as the rubidium source is essential for achieving A-site Rb⁺ doping. Combined with B-site Sn²⁺ doping, this “Rb⁺ and Sn²⁺ co-doping” strategy delivers multiple clear advantages and directly addresses the key pain points mentioned in the background art:1) Strong suppression of parasitic hole-layer emission, improving spectral purity and efficiency
- Problem: Devices made in Comparative Example 1 (Rb⁺ only) and Comparative Example 2 (Sn²⁺ only) both show a pronounced parasitic emission peak at ~443 nm in the electroluminescence spectrum, originating from the hole transport layer (TFB), which reduces color purity and efficiency.
- Result: Devices in Examples 1–4 (Rb⁺, Sn²⁺ co-doped) completely eliminate this parasitic peak (as shown in Fig. 5).
- Mechanism: Co-doping optimizes the QD energy-level structure and, together with the bilayer hole transport design, significantly promotes hole injection and balance from the transport layer into the emissive layer. This prevents interfacial exciton accumulation and quenching, thereby suppressing parasitic emission.
2) Significant improvement in optoelectronic performance
- Substantially higher EQE: Comparative Example 1 (Rb⁺ only) shows an EQE of only 0.9%, while Example 2 (appropriate Rb⁺, Sn²⁺ co-doping) reaches 1.7%. The best-performing device (Example 6, combined with the bilayer transport structure) achieves an EQE as high as 3.16%, representing a multi-fold improvement (see Figs. 7, 9, and Table 1).
- Lower operating voltage and higher brightness: Co-doped devices exhibit lower turn-on voltage and much higher maximum luminance (e.g., Example 6 reaches 508 cd/m²), far exceeding the comparative device (171 cd/m²). This indicates more efficient carrier injection and stronger radiative recombination.
3) Enhanced structural stability and reduced toxicity
- Improved tolerance factor: The ionic radius of Rb⁺ is smaller than that of Cs⁺; incorporating Rb⁺ can tune the perovskite lattice “tolerance factor,” improving crystal structural stability.
- Efficient lead reduction: Introducing Sn²⁺ to partially replace toxic Pb²⁺ significantly reduces lead content while maintaining efficient blue emission, better aligning with environmental requirements. XRD patterns (Fig. 4) show that appropriate co-doping preserves a good cubic-phase structure.
4) Simplified synthesis conditions
- Using starting materials such as rubidium carbonate/rubidium acetate enables the entire QD synthesis to be carried out at room temperature in air, without complicated inert-gas protection or high-temperature hot-injection procedures—simplifying the process, lowering cost, and reducing operational difficulty, which supports industrial scale-up.
Summary
In this invention, rubidium carbonate/rubidium acetate serves as the rubidium source to achieve Rb⁺ and Sn²⁺ co-doping, which is the technical core. Its advantages include:- Performance: Fundamentally resolves the common parasitic hole-layer emission issue in blue perovskite LEDs, greatly improving efficiency, luminance, and spectral purity.
- Materials: Enhances structural stability and effectively reduces lead-related toxicity.
- Process: Enables a simple room-temperature synthesis route.
I. Process Summary
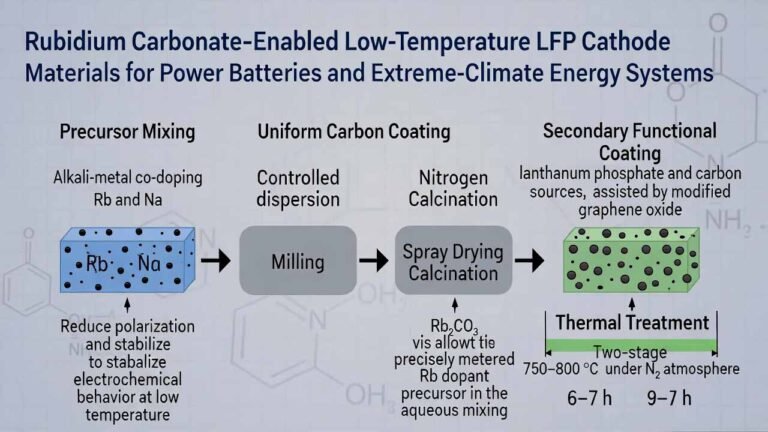
The core innovation of this process is the use of an aqueous solution route to achieve uniform pre-mixing of rubidium. The main steps are as follows:
1) Dissolution and quantification of the rubidium source
- Dissolve rubidium carbonate (or other soluble rubidium salts such as rubidium hydroxide, rubidium nitrate, etc.) in purified water to form an aqueous rubidium salt solution.
- Use an ICP spectrometer to precisely determine the rubidium concentration in the solution, enabling accurate dosing in subsequent steps.
2) Mixing with the precursor
- Based on the target rubidium doping level (calculated as rubidium carbonate, 0.1%–1% of total solid mass), calculate and add the corresponding mass of the ternary precursor NixCoyMz(OH)₂.
- Add additional purified water to adjust the slurry solids content to 50%–70%, forming a uniform solid–liquid mixture.
3) Evaporation and drying
- Continuously stir the solid–liquid mixture at 100–120 °C to evaporate and remove water.
- This yields a mixed powder (Powder B) in which the rubidium salt is uniformly deposited/attached on the surface of precursor particles.
4) Mixing with the lithium source and calcination (sintering)
- Dry-mix Powder B with a lithium source (lithium carbonate or lithium hydroxide) via mechanical mixing, controlling the total molar ratio of Li to transition metals (typically 1.05).
- Perform high-temperature calcination, followed by standard post-processing steps such as crushing, iron removal, and sieving, to obtain the final rubidium-doped ternary cathode material.
Key highlight: Using an aqueous rubidium carbonate solution as the medium and employing a solution impregnation → evaporation-drying approach replaces conventional dry milling or organic-solvent mixing, fundamentally addressing the challenge of uniformly dispersing the rubidium source.
II. Advantages of Using Rubidium Carbonate and This Process
Compared with dry mixing or organic-solvent methods mentioned in the background technology, this method offers the following notable advantages:
1) Solves fundamental dosing and mixing issues for rubidium sources
- Overcomes hygroscopicity effects: Rubidium salts (including rubidium carbonate) are highly hygroscopic; dry weighing can lead to deliquescence, sticking, inaccurate dosing, and agglomeration during mixing. Here, the rubidium salt is prepared as a standard aqueous solution and its concentration is precisely calibrated by ICP, enabling accurate and controllable rubidium doping.
- Achieves near atomic-level uniform dispersion: The aqueous solution can fully wet the precursor particles. Through stirring and evaporation-drying, rubidium ions are uniformly deposited on the precursor surface in solution form, enabling mixing at a molecular/atomic scale and ensuring extremely uniform rubidium distribution in the final product (as reflected by consistent rubidium content across sampling points in the examples).
2) High efficiency, low cost, and improved safety
- No organic solvents needed: This completely avoids the extra cost (e.g., solvent recovery systems), safety risks (flammability/explosion hazards), and reduced throughput (long mixing time, small batch handling) associated with organic media such as ethanol.
- Simple and efficient workflow: Rubidium–precursor mixing is completed via solution treatment, while mixing with the lithium source retains an efficient dry process—resulting in a streamlined overall flow with high productivity.
3) Improved cathode structure and electrochemical performance (expected)
- More regular morphology: SEM images (Figures 1 and 2) indicate intact and regular secondary particle morphology, attributed to uniform liquid-phase mixing that prevents abnormal grain growth caused by local over-doping.
- Foundation for better performance: Uniform rubidium doping is expected to stabilize the crystal structure, suppress phase transitions during charge/discharge, and thereby improve cycling stability, rate capability, and safety. Uniform morphology also benefits electrolyte wetting and lithium-ion diffusion.
Conclusion
The key innovation of this preparation method is the clever use of rubidium carbonate’s water solubility to introduce a critical “aqueous solution impregnation–evaporation drying” step. This effectively resolves the industry-wide issue of inaccurate dosing and non-uniform mixing caused by the hygroscopic nature of rubidium sources. The advantages are reflected not only in an environmentally friendly, safer, efficient, and low-cost process, but also in ensuring a product with high compositional uniformity and well-defined morphology, providing a strong foundation for producing high-performance, highly consistent rubidium-doped ternary cathode materials.
I. Process Summary
The core of this aluminum brazing flux preparation is to use ammonium fluoride (NH₄F) or ammonium bifluoride (NH₄HF₂) as a safer fluorine source, reacting in an aqueous medium with anhydrous rubidium carbonate (Rb₂CO₃), cesium carbonate (Cs₂CO₃), and an aluminum compound (Al₂O₃ or Al(OH)₃) to generate the target fluoride-based flux. The process can be divided into two main routes:
Route 1: One-step Mixing Method
Formulation and dissolution: Dissolve the calculated amount of NH₄F/NH₄HF₂ in water to obtain mixed slurry A.
Mixing: Add pre-dehydrated anhydrous Rb₂CO₃, Cs₂CO₃, and the aluminum compound into slurry A, stir uniformly to obtain mixed slurry B.
Reaction and sintering:
- First, heat with stirring at 90–140°C to dehydrate until the material becomes viscous and non-flowing.
- Then, react at 280–400°C for 1–3 hours to complete the reaction and form the final flux product.
- Post-treatment: Grind the reaction product into powder.
Route 2: Stepwise Reaction Method
Prepare fluorine-source solution and aluminum paste separately: Dissolve NH₄F/NH₄HF₂ in water to obtain the first mixture; mix the aluminum compound with water to form a paste as the second mixture.
Prepare an aluminum fluoride intermediate: Add the first mixture into the second mixture, react first at room temperature and then under heating to boiling. After drying, react at 280–450°C for 1–3 hours to obtain the first powdered material (mainly aluminum fluoride).
Prepare a rubidium/cesium fluoride mixture: Take another portion of the first mixture and react it with anhydrous Rb₂CO₃ and Cs₂CO₃ at room temperature to obtain the fourth mixture (mainly a solution or mixture of rubidium and cesium fluorides).
Mixing and final reaction: Add the first material into the fourth mixture, stir/react at 90–140°C until viscous, then react at 280–350°C for 1–3 hours so that all components fully combine.
Post-treatment: Grind to obtain the final product.
Key Process Control Points
- Raw material dehydration: Cs₂CO₃ and the aluminum compound must be pre-dehydrated at high temperature under an inert atmosphere to ensure an “anhydrous” state and prevent moisture uptake in the final product.
- Accurate stoichiometry: Based on reaction equations, strictly control the molar ratio of the fluorine source to Al, Rb, and Cs (as described in the patent) to ensure complete reaction and avoid high-melting-point byproducts.
- Two-stage heating: The low-temperature stage (90–140°C) is mainly for dehydration and initial reaction; the high-temperature stage (≥280°C) is critical to complete the solid-state reaction, directly impacting the flux melting point and activity.
II. Advantages of Adding Rubidium Carbonate (Rb₂CO₃)
Introducing rubidium carbonate is a key highlight of the patent. Its advantages are mainly reflected in safety/environmental performance, property enhancement, and cost optimization:
1) Improved process safety and environmental friendliness
Replacement of hazardous reagents: Traditional wet preparation of fluoride brazing fluxes often uses hydrofluoric acid (HF), which is highly corrosive and risky, with stringent equipment requirements. This patent uses a metathesis reaction in aqueous solution between Rb₂CO₃ and NH₄F/NH₄HF₂ (forming RbF, CO₂, and NH₃), completely avoiding HF. This greatly improves operational safety, reduces environmental risk, and lowers overall manufacturing cost.
2) Optimized overall flux performance
Synergistic melting point reduction: Rubidium (Rb) and cesium (Cs) are both alkali metals; their fluorides (RbF, CsF) can form low-eutectic systems with aluminum fluoride (AlF₃). By controlling the mass ratio of Rb:Cs:Al to (20–32):(27–32):(10–13), and leveraging the synergy between Rb₂CO₃ and Cs₂CO₃, the flux melting point can be precisely tuned to an ideal medium-temperature brazing range of 460–510°C (suitable for ~500°C brazing), with a narrow melting range and good fluidity.
Enhanced activity and wettability: As indicated by the example data (Table 1), fluxes containing an appropriate amount of Rb₂CO₃ (Examples 1–6) show excellent melting/clarification performance at 510°C (no residue), good spreading area, and strong oxide-removal capability. This suggests that introducing Rb⁺ helps more effectively disrupt the oxide film on aluminum alloy surfaces during brazing, improving wetting and spreading of the filler metal.
Improved moisture resistance and non-corrosive residues: With a well-designed formulation and complete solid-state reaction, the obtained flux is stable. The patent’s moisture-content test shows 0.01%–0.02% water content for Examples 1–6, indicating low moisture absorption (in contrast to Comparative Example 3 using direct mixing of fluorides, which exhibits severe hygroscopicity). Post-brazing residues are non-corrosive (a non-corrosive flux), eliminating the need for post-braze cleaning.
3) Balanced cost and performance
Lower raw material cost: Compared with using expensive finished fluoride salts such as rubidium fluoride (RbF) and cesium fluoride (CsF) directly (e.g., Comparative Example 3), using relatively lower-cost carbonates (Rb₂CO₃, Cs₂CO₃) and generating the required fluorides in situ via reaction with ammonium fluoride significantly reduces raw material costs.
Avoids hygroscopic fluoride-salt handling issues: RbF and CsF are highly hygroscopic, making storage and weighing difficult and affecting stoichiometric accuracy. Using carbonate forms is easier to store and weigh precisely, improving process quality control.
Summary Comparison
| Feature | This invention (with Rb₂CO₃) | Traditional / comparative methods | Key advantage |
| Raw materials & safety | Uses Rb₂CO₃ + NH₄F/NH₄HF₂; avoids HF | Often uses HF, or directly uses hygroscopic RbF/CsF | Safer, more environmentally friendly; easier storage |
| Process | Wet reaction + high-temp sintering; simple and controllable | HF reaction is highly risky, or simple physical mixing | Suitable for scale-up; good quality control |
| Performance | Melting point 460–510°C; clean melting, good spreading; low moisture uptake | Melting point may be higher (e.g., Comp. Ex. 4), or severe hygroscopicity/low activity (e.g., Comp. Ex. 3) | Balanced performance; suitable for medium-temp Al brazing |
| Cost | Carbonates + ammonium fluoride; lower cost | HF (high safety cost) or finished RbF/CsF (high raw material cost) | Clear cost advantage |
Conclusion: By introducing rubidium carbonate and reacting it (together with cesium carbonate and aluminum compounds) with NH₄F/NH₄HF₂ at specific ratios, this patent innovatively develops a safe, low-cost, high-performance aluminum brazing flux preparation method. The addition of Rb₂CO₃ is not only essential for enabling a safer preparation route, but—together with cesium—also serves as the key factor for precise melting-point control and enhanced oxide-removal, wetting, and moisture-resistance performance.
Safety Information
| Information | |
|---|---|
| Chemical Formula | Rb2CO3 |
| Molecular Weight | 230.945 g/mol |
| Appearance | White powder, very hygroscopic |
| Storage | Store in a cool, dry place away from incompatible materials like acids |
| Hazard Classification | Irritant, Harmful if swallowed, May cause respiratory irritation |
| PPE Required | Safety goggles, Lab coat, Gloves |
| Primary Uses | Glass-making, catalyst for short-chain alcohols, preparation of rubidium metal and salts |
| Hazards | Skin, eye, and respiratory irritant |
| First Aid Measures | Provide fresh air, rinse with water, seek medical advice |
| Firefighting Measures | Use CO2, extinguishing powder, water spray; releases CO and CO2 fumes in fire |
| Accidental Release Measures | Use PPE, ensure ventilation, prevent environmental release |
| Handling Precautions | Handle under dry protective gas, avoid moisture, ensure good ventilation |
| Storage Conditions | Store in cool, dry place away from water/moisture and strong bases |
| Personal Protective Equipment | Respirators, impervious gloves, safety glasses, protective clothing |
| Physical Properties | Odorless white powder, insoluble in water, high solubility rate |
| Chemical Stability | Stable under recommended conditions, avoid water/moisture and bases |
| Toxicological Information | Causes skin and eye irritation, respiratory irritation; no known carcinogenic effects |
| Ecological Information | Avoid release into environment; no data on aquatic toxicity |
| Disposal Considerations | Dispose according to official regulations |
| Transport Information | Not listed as marine pollutant or under specific UN transport classifications |
| Regulatory Information | Complies with U.S. EPA Toxic Substances Control Act, Canadian NDSL, not listed under California Proposition 65 or REACH |
Potassium Carbonate vs Rubidium Carbonate
K2CO3 and Rb2CO3 are both group-1 carbonates (ionic M₂CO₃ salts). They behave similarly as carbonate bases, but differ in solubility, handling, price, and where they deliver value.
| Aspect | Potassium carbonate (K₂CO₃) | Rubidium carbonate (Rb₂CO₃) |
|---|---|---|
| Overall role | General, cost‑effective base and glass modifier for bulk applications | High‑end functional material for niche, performance‑critical applications |
| Glass clarity | Provides high transparency and low bubble content, sufficient for most optical/industrial glass | Enables very high optical quality in specialty glasses when extreme performance is needed |
| Thermal performance in glass | Significantly improves heat resistance and thermal shock resistance | Further raises softening and transition temperatures for extreme thermal stability |
| Mechanical/chemical durability | Increases strength and chemical resistance to meet daily and industrial needs | Contributes to long‑term stability in demanding optical/electronic environments |
| Optical performance (refractive) | Provides adequate refractive index for common optical and display glasses | Enables higher refractive index and dispersion for advanced lenses and optoelectronic uses |
| Electrical properties in glass | Suitable for ordinary insulation requirements | Helps reduce electrical conductivity for high‑voltage, high‑field insulation glass |
| Effect as base/catalyst | Medium‑strong base; stable, easy to scale up, side reactions relatively easy to control | Often higher solubility and stronger effective basicity; can give higher conversion/selectivity in specific reactions |
| Process robustness | Wide operating window; tolerant to fluctuations, low process‑tuning cost | Used in tightly controlled, precision processes; rewards strict control with superior performance |
| Economic effect | Very good cost‑performance ratio; ideal for large‑volume production | High cost; only worthwhile when performance gain is critical for product specifications |
| Typical use level | Bulk chemical, ton‑scale usage | Small‑scale, high‑value usage in research and high‑tech manufacturing |
Why Choose Our Rubidium Carbonate
As a professional direct manufacturer of rubidium carbonate (Rb₂CO₃), we control the complete vertical supply chain from upstream to downstream, ensuring exceptional product purity, reliable supply stability, and customized solutions. Here are our core competitive advantages:
Is rubidium carbonate soluble in water?
Rubidium carbonate is highly soluble in water.
- Qualitative description: Listed as “soluble” or “very soluble” in water in chemical databases.
- Quantitative solubility: Approximately 4500 g/L at 20°C — an extremely high value for an inorganic salt (this figure is consistently reported across chemical suppliers, safety data sheets, and databases).
- Solution properties: A 50 g/L aqueous solution has pH ≈ 11.7, indicating a strongly alkaline solution due to hydrolysis of the carbonate ion.

Frequently Asked Questions
Most frequent questions and answers
How can I purchase Rubidium Carbonate from your company?
You can purchase Rubidium Carbonate by visiting our website and selecting the desired quantity. Alternatively, you can contact our sales team directly for a quotation and to discuss specific requirements.
What packaging options are available for Rubidium Carbonate?
We offer various packaging options to suit different needs, including standard industry drums, bags, and custom packaging upon request. All packaging ensures safe and compliant transportation.
Is there a minimum order quantity for Rubidium Carbonate?
Yes, there is a minimum order quantity, which can vary depending on the product grade and packaging. Please contact our sales team for more details.
Do you provide samples of Rubidium Carbonate for testing?
Yes, we can provide samples for testing purposes. Please contact our sales team to request a sample and discuss any specific requirements.
Can I get a discount for bulk purchases of Rubidium Carbonate?
Yes, we offer discounts for bulk purchases. The discount rate depends on the quantity ordered. Please contact our sales department for a detailed quote.
What payment methods do you accept for purchasing Rubidium Carbonate?
We accept various payment methods, including bank transfers, PayPal, and, in some cases, letters of credit. Specific payment terms can be discussed during the order process.