Rubidium Chloride (RbCl)
Synonyms: Rubidium chloridee, Rubidium Salt
CAS Number: 7791-11-9 , EC Number:232-240-9
Brand Name : DQ
Percentage assay:99.5%
Package Information: Bottle or 25kg/drum or customized packing
Rubidium Chloride Price
Product Information
Impurity Content (Mass Fraction)/%, Not Exceeding
| Product Grade | Li | Na | K | Cs | Ca | Mg | Fe | Al | Si | Pb |
|---|---|---|---|---|---|---|---|---|---|---|
| 99.0% | 0.0010 | 0.010 | 0.050 | 0.20 | 0.0050 | 0.0010 | 0.0010 | 0.0010 | 0.0010 | 0.0010 |
| 99.5% | 0.0010 | 0.010 | 0.050 | 0.10 | 0.0050 | 0.0010 | 0.0005 | 0.0010 | 0.0010 | 0.0005 |
| 99.9% | 0.0005 | 0.0050 | 0.010 | 0.030 | 0.0020 | 0.0005 | 0.0005 | 0.0005 | 0.0005 | 0.0005 |
| Parameter | Value or Description | Units or Conditions | Relevance or Application |
|---|---|---|---|
| Molecular Weight | 120.92 g/mol | Grams per mole | Basic Chemistry |
| Melting Point | 718 °C | Celsius | Material Properties |
| Boiling Point | 1,390 °C | Celsius | Material Properties |
| Density | 2.80 g/cm³ | Grams per cubic centimeter | Material Properties |
| Solubility | Highly soluble in water | N/A | Chemical Properties |
| Appearance | White or colorless crystalline solid | N/A | Physical Properties |
| Electrical Conductivity | High | Siemens per meter (S/m) | Electrical Properties |
| Chemical Stability | Stable under standard conditions | N/A | Storage and Handling |
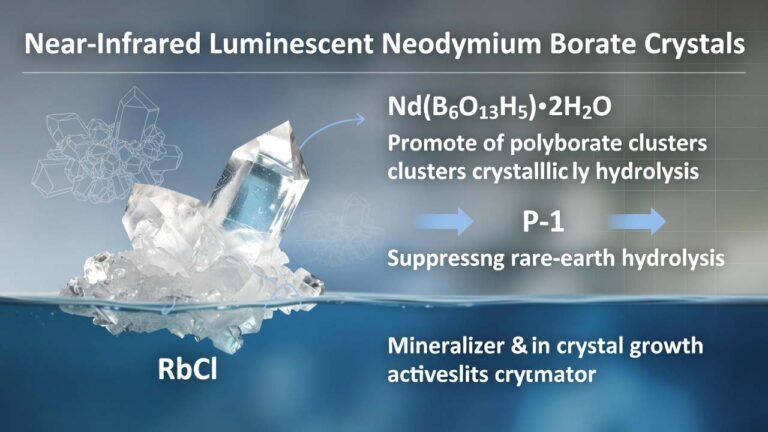
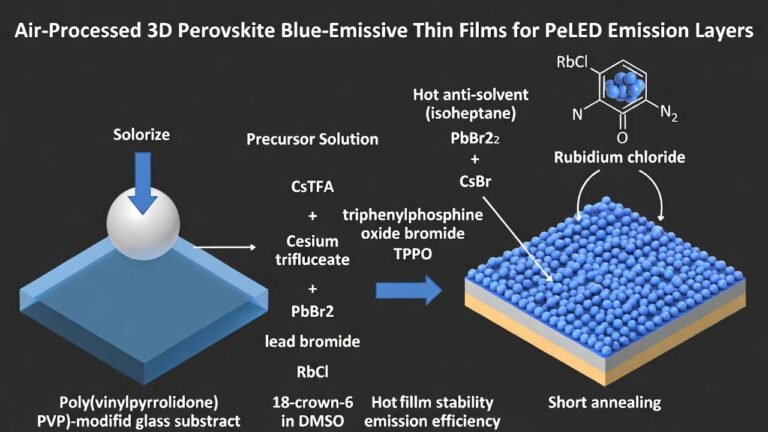
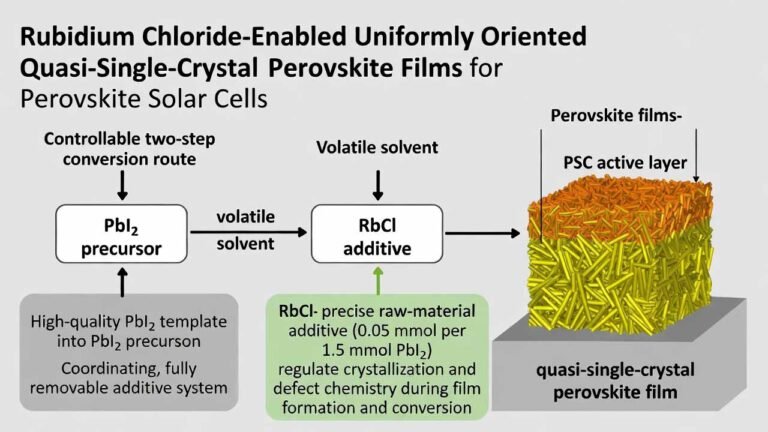
| Common Uses | Research, catalyst, medicine | N/A | Industrial Applications |
Rubidium Chloride Formul
| Reaction Description | Balanced Equation | Reaction Conditions | Application or Significance |
|---|---|---|---|
| Formation from elements | Rb(s)+Cl2(g)→RbCl(s) | Standard conditions | Synthesis of RbCl |
| Dissolution in water | RbCl(s)→Rb+(aq)+Cl−(aq) | Aqueous solution | Solubility |
| Reaction with Sodium Sulfate | RbCl(aq)+Na2SO4(aq)→2NaCl(aq)+Rb2SO4(s) | Aqueous solution | Precipitation reaction |
| Reaction with Silver Nitrate | RbCl(aq)+AgNO3(aq)→RbNO3(aq)+AgCl(s) | Aqueous solution | Precipitation of AgCl |
Applications of Rubidium Chloride
| Application Area | Description | Industries Involved | Relevance to Your Business |
|---|---|---|---|
| Biological Research | Used in molecular biology to precipitate DNA and in the isolation of plasmid DNA. | Biomedical, Research | Biomedicine |
| Electrolyte in Batteries | Due to good ionic conductivity, used as an electrolyte in certain batteries. | Energy, Automotive | Electric Power, Battery |
| Chemical Synthesis | Starting material for the synthesis of other rubidium compounds. | Chemical Manufacturing | Catalyst |
| Medicine | Used as a biomarker to study the behavior of potassium channels in the heart. | Healthcare, Pharmaceutical | Biomedicine |
| Spectroscopy | Used in certain types of spectroscopy for molecular structure analysis. | Research, Academic | Quantum Computing |
| Catalysis | Used in some catalytic reactions, particularly in organic synthesis. | Chemical Manufacturing | Catalyst |
| Glass Manufacturing | Used in special types of glass where high refractive index or other properties are required. | Manufacturing | Special Glass |
| Fireworks | Rubidium compounds, including RbCl, can give fireworks a purple color. | Entertainment | N/A |
| Semiconductor Industry | Used in processes for the preparation of special semiconductor materials. | Electronics, Technology | N/A |
| Specialized Alloys | Used in the preparation of certain specialized alloys. | Metallurgy | N/A |
| Atomic Clocks | Rubidium isotopes obtained are used in highly accurate atomic clocks. | Timekeeping, Navigation | Atomic Clock |
| Quantum Computing | Rubidium isotopes derived are used in experiments related to quantum computing. | Technology, Research | Quantum Computing |
Safety Information
| Safety Category | Description | Precautionary Measures | Emergency Response |
|---|---|---|---|
| Physical Hazards | Non-flammable, non-explosive. | Store in a cool, dry place. | N/A |
| Health Hazards | May cause skin and eye irritation. Can be harmful if swallowed or inhaled. | Wear protective clothing, gloves, and eye/face protection. | Wash with plenty of water, seek medical advice. |
| Environmental Hazards | May be harmful to aquatic life. | Avoid release to the environment. | Contain spillage, and then collect with absorbent material. |
| Chemical Hazards | Reacts with strong acids to produce toxic fumes. | Store away from strong acids. | Use water spray to dilute fumes. Evacuate area. |
| First Aid Measures | In case of skin contact, wash with plenty of water. If ingested, seek medical advice immediately. | Keep first aid kit nearby. | Provide medical attention immediately. |
| Storage Requirements | Store in a cool, dry place away from incompatible materials like strong acids. | Properly label storage containers. | In case of spillage, clean immediately. |
| Disposal Considerations | Dispose of in accordance with local, state, and federal regulations. | Use authorized waste disposal sites. | Handle waste material as hazardous waste. |
| PPE (Personal Protective Equipment) | Use gloves, safety goggles, and lab coat during handling. | Ensure proper ventilation. | In case of exposure, remove contaminated clothing. |