Rubidium Iodide (RbI) Additive Electrolyte for Aqueous Zinc-Ion Batteries: Dendrite Suppression, Longer Cycling, and Safer Cells
1) Overview and Technical Value
Aqueous zinc-ion batteries (AZIBs) are attractive for large-scale energy storage because they use non-flammable water-based electrolytes and rely on abundant zinc resources. However, Zn metal anodes are highly reactive in aqueous environments, often suffering from dendrite growth, “dead Zn” formation, hydrogen evolution reaction (HER), and corrosion. These issues can accelerate polarization rise, reduce reversibility and energy efficiency, and in practical cells contribute to swelling, leakage, and short-circuit risks.
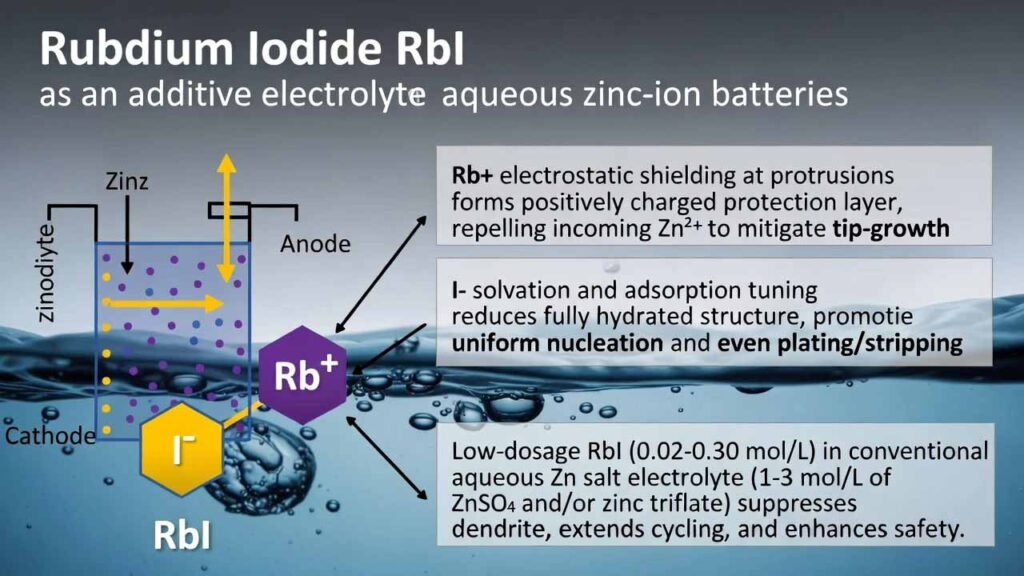
This electrolyte strategy introduces rubidium iodide (RbI) as a low-dosage additive (0.02–0.30 mol/L) into a conventional aqueous Zn salt electrolyte (1–3 mol/L of ZnSO4 and/or zinc triflate). RbI delivers a dual-ion interface regulation effect:
- Rb+ “electrostatic shielding” at protrusions: Rb+ preferentially adsorbs on dendrite tips/convex sites and forms a positively charged protection layer that repels incoming Zn2+, mitigating the tip-growth (“needle”) effect and guiding smoother deposition.
- I− solvation and adsorption tuning: I− can participate in Zn2+ solvation (reducing the fully hydrated structure) and preferentially adsorb on Zn surfaces, accelerating uniform nucleation and promoting even plating/stripping.
For R&D teams, the technical value is that RbI enables a simple, scalable electrolyte formulation that targets the root cause (unstable Zn/electrolyte interface), improving cycling stability and safety without complicated electrode remodeling.
2) Detailed Experimental Process
2.1 Materials and Target Formulations
- Solvent: Ultrapure water, resistivity 18–25 MΩ·cm.
- Zinc salt electrolyte: Total concentration 1–3 mol/L (commonly 2.0 mol/L ZnSO4 shown in the example).
- Additive: Rubidium iodide (RbI) at 0.02–0.30 mol/L (frequently highlighted working window 0.05–0.30 mol/L).
- Representative “best-performing” example: 2.0 M ZnSO4 + 0.05 M RbI.
Practical note for formulation work: use analytical-grade salts, keep containers tightly capped (iodide salts can be moisture sensitive), and filter if needed for high-precision electrochemistry.
2.2 Electrolyte Preparation Procedure
- Prepare Zn salt solution: Add the zinc salt (e.g., ZnSO4·7H2O) into ultrapure water to reach the target concentration (e.g., 2.0 mol/L).
- Stir to dissolve: Magnetically stir until fully dissolved, then continue mixing and allow it to stand overnight.
- Rest/settle: After overnight mixing, keep the solution static for ~12 hours to obtain a stable electrolyte base.
- Add RbI: Dose RbI into the Zn salt solution to the desired additive level (e.g., 0.05 mol/L).
- Final mixing: Stir until RbI is completely dissolved and the electrolyte is homogeneous. Label and store in a sealed bottle.
2.3 Coin Cell Assembly (CR2032) and Testing Workflow
-
Electrode & separator preparation:
- Zn foil (anode): thickness 0.01 mm, punched to 12 mm diameter.
- Counter electrode options: copper foil thickness 0.002 mm, punched to 12 mm; or stainless steel for certain asymmetric tests.
- Separator: glass fiber, punched to 16 mm diameter.
- Symmetric cell (Zn || Zn): Assemble Zn foil on both sides with glass fiber separator in between. Add electrolyte volume ~80 μL.
- Asymmetric cell (Zn || Cu or Zn || SS): Use Zn foil as the plating/stripping electrode and Cu (or SS) as the counter/collector side. Add electrolyte ~80 μL.
- Sealing: Place gasket/spacer/spring as standard for CR2032 and crimp with a coin-cell sealer.
- Electrochemical tests (typical conditions): Galvanostatic plating/stripping at current density 1 mA·cm⁻² and areal capacity 1 mAh·cm⁻². Track overpotential evolution, voltage fluctuation, short-circuit behavior, and Coulombic efficiency (for Zn || Cu).
| Example Electrolytes for Benchmarking | Purpose of Comparison |
|---|---|
| 2.0 M ZnSO4 | Baseline aqueous Zn salt electrolyte (no additive). |
| 2.0 M ZnSO4 + 0.05 M RbI | Target formulation: dual-ion interface control (Rb+ + I−). |
| 2.0 M ZnSO4 + 0.05 M Rb2SO4 | Rb+-only effect (no iodide) to test electrostatic shielding alone. |
| 2.0 M ZnSO4 + 0.05 M ZnI2 | I−-only effect (no rubidium) to test iodide regulation alone. |
3) Comparison Summary: This Production Route vs. Traditional Approaches
From a manufacturing and lab-scale reproducibility perspective, adding RbI to an aqueous Zn salt electrolyte is a “low-disruption” route: it preserves the core electrolyte chemistry while improving interfacial behavior through controlled adsorption and solvation tuning. Compared with common Zn-anode improvement strategies, the differences are clear:
Electrolyte Additive Route (RbI)
- Process simplicity: dissolve → stir overnight → rest 12 h → add RbI → stir.
- Scalability: compatible with standard electrolyte blending tanks and QA protocols.
- Interface-first improvement: targets dendrites/HER/corrosion at the Zn/electrolyte boundary without redesigning electrodes.
- Performance signal: reduced initial polarization and stabilized voltage profiles indicate more uniform nucleation and plating.
Traditional Routes (Coatings / Structured Anodes)
- Higher process complexity: coating deposition, curing, multi-step surface treatments, or 3D host fabrication.
- Cost and labor: more equipment steps and tighter process control needed.
- Variable effectiveness: improvements may be limited if local ion flux remains non-uniform during cycling.
- Integration burden: electrode modifications may require re-qualification in cell assembly and manufacturing.
In head-to-head additive comparisons, the dual-ion nature of RbI is the key differentiator: Rb+-only salt (e.g., Rb2SO4) may provide partial shielding but is typically less effective, while I−-only additive (e.g., ZnI2) can help solvation/adsorption but lacks the strong “tip-site” electrostatic suppression contributed by Rb+. Combining both in one salt (RbI) creates a more complete interface regulation package.
4) Why Rubidium Iodide (RbI) Excels in This Application
4.1 Mechanistic Advantages (R&D Perspective)
- Rb+ is not consumed during cycling: its reduction potential is far below Zn2+, so it does not plate out on the anode. It stays available to adsorb and regulate the interface over long cycles.
- Selective adsorption at protrusions: Rb+ concentrates on convex/tip regions and creates a positively charged “shielding shell,” repelling Zn2+ and redirecting deposition toward flatter regions for smoother growth.
- Solvation structure tuning by I−: iodide can partially replace water in the Zn2+ solvation sheath, lowering the penalty for desolvation and enabling more uniform nucleation/kinetics.
- Synergistic interface stabilization: I− supports uniform nucleation and transport, while Rb+ suppresses tip-driven amplification. Together they reduce dendrites, dead Zn, hydrogen evolution, and corrosion.
4.2 Engineering Benefits You Can Validate in Standard Tests
- Lower initial polarization: indicates faster, more homogeneous nucleation and reduced local current hotspots.
- Longer symmetric-cell life: stable plating/stripping for extended hours suggests effective dendrite suppression and fewer parasitic reactions.
- Improved Coulombic efficiency in Zn || Cu: higher and steadier CE implies better reversibility and less “dead Zn.”
- Cleaner post-mortem morphology: Zn surfaces show denser, more uniform deposits instead of porous byproducts and dendritic protrusions.
Reported benchmark signals under 1 mA·cm⁻² and 1 mAh·cm⁻² include substantially extended cycling for RbI-containing electrolytes, with an observed performance peak around 0.05 mol/L RbI in a 2.0 mol/L ZnSO4 electrolyte.
4.3 Practical Formulation Guidance (For Development Teams)
- Start with a concentration sweep: 0.02, 0.05, 0.10, 0.20, 0.30 mol/L RbI to map the best window for your electrode/separator system.
- Use consistent water quality: keep ultrapure water resistivity in the stated range to avoid introducing uncontrolled side reactions.
- Keep assembly consistent: identical Zn foil thickness, separator type, and electrolyte volume (~80 μL) to isolate additive effects.
- Benchmark against single-ion controls: compare to an Rb+-only salt and an I−-only salt to confirm true synergy.
Result: By positioning rubidium iodide as the key raw-material additive in aqueous Zn electrolytes, this approach offers a practical route to suppress dendrites and “dead Zn,” reduce HER/corrosion, extend cycle life, and enhance safety margins for aqueous zinc-ion batteries and related Zn-based electrochemical energy storage devices.
The mentioned synthesis method references patent document number CN202311262111.5