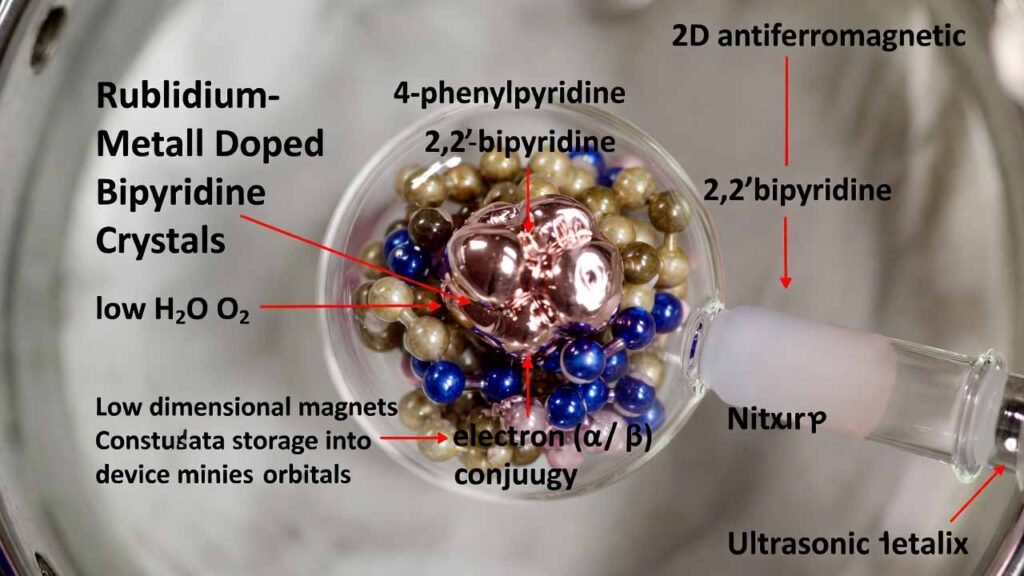
Rubidium-Metal Doped Bipyridine Crystals for 2D Organic Antiferromagnets
A practical, engineer-friendly workflow to embed highly reducing rubidium metal (Rb) into bipyridine-family molecular crystals and drive low-dimensional magnetism through controlled electron transfer into conjugated (τ / π) orbitals.
1) Overview and technical value
Low-dimensional magnets are attractive for next-generation information technologies because magnetic order can persist in extremely thin geometries, enabling dense data storage concepts, device miniaturization, and new quantum/spintronic functionalities. A major bottleneck is that many established low-dimensional magnets are inorganic and/or rely on complex fabrication routes (e.g., exfoliation, epitaxy), while purely organic low-dimensional magnets based on s/p-electron spins remain comparatively rare.
This workflow targets an organic route: combine bipyridine-class molecules (e.g., 4-phenylpyridine, 2,2'-bipyridine, 2,4'-bipyridine) with rubidium metal under ultralow H2O/O2 conditions, then promote controlled solid-state doping inside a sealed quartz ampoule using a constant-temperature ultrasonic bath. The key technical value is that Rb acts as an electron source, allowing its valence electron(s) to be injected into the conjugated orbital manifold of the organic crystal, forming a low-dimensional magnetic lattice (commonly observed as 2D antiferromagnetic behavior in magnetic measurements).
- Core mechanism Rubidium metal donates electrons; the organic framework hosts the injected electrons, enabling low-dimensional magnetic exchange.
- Process simplicity One sealed-tube step (ultrasonic, mild temperature) replaces multi-step thin-film growth routes.
- Engineering control Doping and crystallinity are tuned by stoichiometry, temperature window, sonication time, and cooling profile.
2) Detailed experimental procedure
Materials
- Bipyridine-class molecule (choose one): 4-phenylpyridine, 2,2'-bipyridine, or 2,4'-bipyridine
- Rubidium metal (Rb) (freshly cut/cleaned; handled under inert atmosphere)
- Quartz tube/ampoule suitable for high-vacuum sealing
- Calcium chloride (CaCl2) aqueous solution for elevated-temperature bath medium
Equipment
- Glovebox (H2O and O2 both < 0.1 ppm)
- High-vacuum pump system capable of < 1×10-4 Pa
- Quartz tube sealing setup (flame sealing / melt sealing)
- Temperature-controlled ultrasonic bath with cover
- Characterization: XRD; magnetometer (e.g., PPMS) for ZFC/FC and M–H curves
Step-by-step workflow
-
Inert-atmosphere preparation
In a glovebox where H2O and O2 are both < 0.1 ppm, weigh the chosen bipyridine-class molecule and rubidium metal. Use an atomic molar ratio in the range of (molecule):(Rb) = 1–2 : 1–2. A common starting point is 1:1. -
Mixing and loading
Gently mix the solids (avoid friction/heat). Load the mixture into a clean, dry quartz tube. Keep exposure to air/moisture strictly avoided throughout. -
High-vacuum evacuation and sealing
Connect the quartz tube to a vacuum line and evacuate to < 1×10-4 Pa. Once stable, flame-seal (melt-seal) the tube to obtain a fully sealed ampoule. -
Ultrasonic, constant-temperature bath treatment
Place sealed ampoules into a temperature-controlled ultrasonic bath filled with CaCl2 aqueous solution. Heat from ambient to target temperature within about 30 minutes, then sonicate at constant temperature:- Temperature: 85–100 °C (typical setpoints: 85 °C or 98 °C depending on the molecule)
- Time: 8–12 hours (typical: ~10 hours)
- Bath: keep covered to stabilize temperature and minimize evaporation
-
Controlled cooling and recovery
After sonication, cool slowly in the bath. Remove ampoules after the system cools to < 60 °C. Typical natural cool-down to <60 °C takes ~45–70 minutes depending on the setpoint and bath load. For process control, an optional programmed cooling profile (e.g., 2 °C/h down to <60 °C) can be used. -
Product handling
Open ampoules only in an inert atmosphere (glovebox) and isolate the doped molecular crystals. Store samples air-free to prevent degradation and side reactions.
Recommended parameter window (engineering targets)
| Control variable | Working window | Why it matters |
|---|---|---|
| H2O / O2 | < 0.1 ppm / < 0.1 ppm | Prevents rapid quenching of Rb reactivity; avoids parasitic products (e.g., hydrides/hydroxides) and preserves electron-transfer efficiency. |
| Vacuum level | < 1×10-4 Pa | Removes residual gases and moisture, stabilizes sealed-tube chemistry, improves reproducibility and crystallinity. |
| Bath temperature | 85–100 °C | Too low: incomplete doping; too high: organic decomposition risk and increased side-product formation. |
| Sonication time | 8–12 h | Too short: incomplete reaction and residual starting materials; too long: higher decomposition probability and reduced crystal quality. |
| Cooling profile | Slow cool to <60 °C | Rapid quenching can suppress crystallization and increase amorphous content; controlled cooling supports crystal growth. |
Quality checks (typical)
- XRD: disappearance/weakening of starting-material peaks and emergence of new diffraction peaks indicates formation of a new crystalline phase.
- Magnetometry (PPMS): ZFC/FC susceptibility curves that overlap across broad temperature windows are consistent with antiferromagnetic/low-dimensional behavior; sample-dependent peaks can appear near characteristic temperatures.
- M–H curves: linear M–H at selected temperatures helps exclude superparamagnetism/spin-glass signatures and supports antiferromagnetic interpretation.
Safety note (engineering minimum): rubidium metal is pyrophoric/water-reactive. Only handle under inert atmosphere with appropriate PPE, shielding, and trained procedures. Never introduce water to rubidium residues.
3) Comparison to traditional routes (summary for scale-up decisions)
Conventional low-dimensional magnet preparation often depends on thin-flake isolation (mechanical exfoliation) or controlled growth methods (various epitaxial or transport techniques). These can yield high-quality specimens but may face scale, throughput, or uniformity constraints. In contrast, this sealed-tube ultrasonic approach focuses on bulk molecular crystal doping using rubidium metal as the active electron donor.
| Route | Typical requirements | Common constraints | How the Rb-doped sealed-tube workflow differs |
|---|---|---|---|
| Mechanical exfoliation / flake isolation | Layered crystals; handling, transfer, thickness selection | Low yield, small area, sample-to-sample variability | Produces bulk doped molecular crystals; tuning is via stoichiometry + thermal/sonic profile rather than flake selection |
| Epitaxial / specialized thin-film growth | High-vacuum systems, substrates, growth optimization | High equipment cost; process complexity; material compatibility limits | Uses standard glovebox + vacuum sealing + ultrasonic bath; no substrate dependence |
| Organic magnet routes using paramagnetic metals or radicals | Metal-ligand design or radical stabilization chemistry | Material-specific synthesis; stability challenges; not always low-dimensional | Targets low-dimensionality through Rb electron injection into conjugated orbitals and layered packing in bipyridine derivatives |
Practical takeaway: if your goal is a scalable pathway to organic low-dimensional magnet candidates with strong charge-transfer control, rubidium metal doping in a sealed ampoule can be a simpler production-style route than device-oriented thin-film methods.
4) Why rubidium metal is the critical raw material advantage
The performance of this application is tied directly to the chemical identity of the dopant. Rubidium metal is not a passive additive here—it is the electron source that enables the magnetic ground state by driving charge transfer into the organic framework.
-
High electron-donation capability
Rubidium readily provides valence electron density to the conjugated orbital system, enabling the formation of an electron-rich molecular crystal where magnetic exchange can emerge from s/p-electron spins. -
Efficient doping into layered molecular packing
Bipyridine/pyridine rings are relatively electronegative, which can lower the reaction barrier with Rb and improve charge-transfer efficiency, helping Rb distribute both within molecular layers and between layers—useful for 2D exchange pathways. -
Process tunability through Rb stoichiometry
The (molecule):(Rb) ratio (e.g., 1:1 as a baseline, expanded to 2:1 or 1:2) gives a direct engineering knob to tune carrier density, phase formation, and magnetic coupling. -
Cleaner magnetic interpretation when Rb-driven charge transfer is controlled
When oxygen/water are suppressed and time/temperature are kept in-window, the product quality improves and side phases are minimized, making XRD and magnetometry conclusions more reliable for R&D iteration. -
Manufacturing implication
Because rubidium is extremely moisture sensitive, consistent results depend on high-purity Rb metal supply, appropriate packaging (sealed, inert), and well-defined handling procedures. Treat Rb purity and surface condition as first-class process variables.
Engineer’s troubleshooting rule-of-thumb: if you see incomplete conversion (residual starting-material peaks), raise sonication time within the 8–12 h window or optimize bath temperature toward the molecule-specific optimum. If you see decomposition/side phases, reduce temperature or time and tighten moisture/oxygen control.
References (for deeper reading)
- Takabayashi et al., Nature Chemistry (2017): π-electron quantum spin-liquid state in ionic polyaromatic hydrocarbons (alkali-metal doped molecular solids).
- Low-dimensional molecule-based / metal–organic magnets and quantum models overview (review literature).
- Rubidium metal SDS and pyrophoric handling procedures (institutional EHS guidance).
- 2D materials and 2D magnet preparation routes; limitations of exfoliation-based workflows (review literature).
- Boiling-point elevation in CaCl2 brines (engineering references) supporting stable high-temperature bath operation.